Scientific Research Results1. Basic regularities of the formation, composition, structure and some properties of PEO-layers on aluminum, titanium in electrolytes with polyphosphate complexes of M(II), M(III) have been determined. The conditions of the obtaining of some surface structures, containing oxide of the metal under treatment, as well as some controlled quantity of the compounds of M(II), M(III) (oxides, phosphates, and spinels) have been substantiated. |
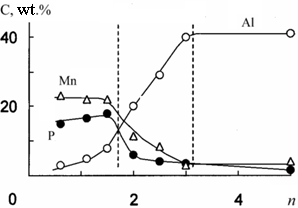
Characteristic dependence of the elemental composition of PEO-layers on aluminum on molar ratio (n = [polyphosphate] / [M]) in electrolyte |
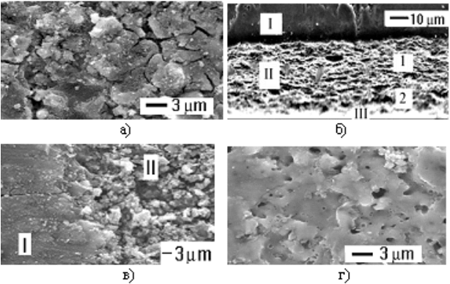 Manganous oxides |
Table: Phase composition of the layers, formed in electrolytes with polyphosphate complexes Ni(II), Mg(II) and Zr(IV)
| Metal under the treatment | Phase composition of the coating after the air annealing at 800оС |
| Ti | NaTi2(PO4)3, Na4(TiO)(PO4)2, Ni0.5(TiO)(PO4), Ni2P2O7, TiO2(a) |
| Nb | Nb2O5, NbO(PO4), Ni2P2O7 |
| Al | Al2O3, NiAl2O4, Ni2P2O7 |
| Zr | ZrO2(c), ZrO2(m), NaMgPO4 |
| Ti | TiO2(a, r), Ti0,8Zr0,2P2O7, ZrP2O7 |
|
Field of application of coatings containing phosphates of M(II), M(III) or M(IV): Catalysts and catalyst carriers (AlPO4, phosphates of rare earth elements, vanadium phosphates, ZrP2O7 и др.) |
Colored, decorative coatings |
 |
Protective phosphate alloys, phosphate ground (zinc, magnesium, iron phosphates etc.) |
Heat-resistant layers (phosphates Zr, Hf etc.) |
Adsorbents (magnesium and calcium phosphates etc.) |
|
Ability for luminescence at the addition of activators |
Layers with bactericidal properties (zinc, copper and silver phosphates etc.) |
Layers, compatible with biological objects (calcium phosphates etc.) |
2. Basic regularities of the formation, composition, structure and some properties of PEO-layers on aluminum, titanium in isopoly and heteropoly electrolytes have been established. The conditions of the obtaining of some layers, containing oxides of tungsten, molybdenum, vanadium and so on have been substantiated.
PEO-layers on aluminum, formed in tungstate-borate electrolytes at pH below and above 7.5. At pH<7.5 electrolyte contains [BW11O39H]8-.

рН < 7,5 |

рН > 7,5 |
Some perspective arrears of application:
 |
Protective layers |
Catalysis |
|
Other functional properties |
3. Basic regularities of the formation, composition, structure and some properties of PEO-layers on aluminum, titanium in electrolytes with fluoride complexes of transition metals have been also determined. The conditions of the obtaining of some layers with oxides of zirconium, titanium, hafnium, niobium on aluminum have been substantiated. |
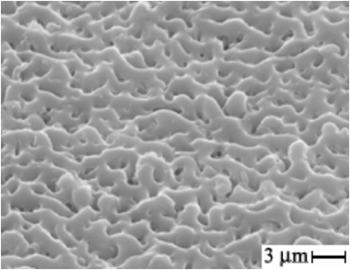
The organization of coating containing ZrO2 on aluminum. |
Some possible fields of application: light-reflection, a protection of corroding medium and of thermal influence. 4. The use of the electrolytes, producing precipitates, for receiving PEO-layers of necessary composition has been determined. For example, in alkaline electrolyte, producing some particles of manganese hydroxide, the layers containing the oxides Mn2O3 and Mn3O4 on the surface have been obtained. 5. Some results on the mechanism of PEO-layers growth under the effect of assembly of independent spark and arc discharges, by means of formation and radial growth of the new phase nuclei as well as the action of the spreading electric discharges have been received. |
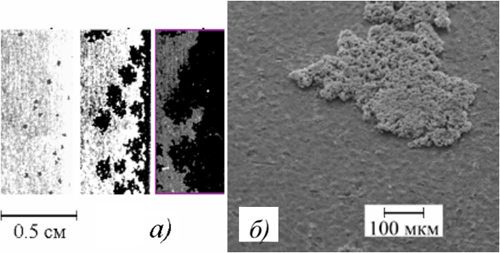
PEO-layer growth by means of formation and radial growth of the new phase nuclei |
6. Some investigations of the catalytic activity of the synthesized structures are being carried out. All the results obtained show that the PEO-method is advantageous to the preparing of catalytic metal/oxide compositions, complex sandwich structures in order to oxidation of CO, and later on of fuel, for example in internal-combustion engine. 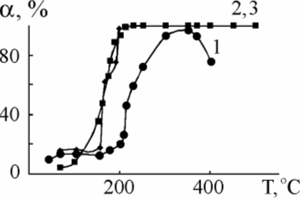
The CO conversion (αСО (%)) vs. reaction temperature at Ti/TiO2/MnOx. Silver content С(Ag), ат.%: 1 - 0; 2 - 4.6; 3 - 7.1. |